As we all enter our third year of living alongside the COVID-19 pandemic, many aspects of our daily lives have been left irrevocably changed. From social distancing to mask mandates, stepping out our front doors is a completely different experience as compared to a year prior. And that reality is only further compounded by the use of COVID-19 self test kits.
Made-in-Malaysia COVID test kit scores 100% accuracy rate in international assessment
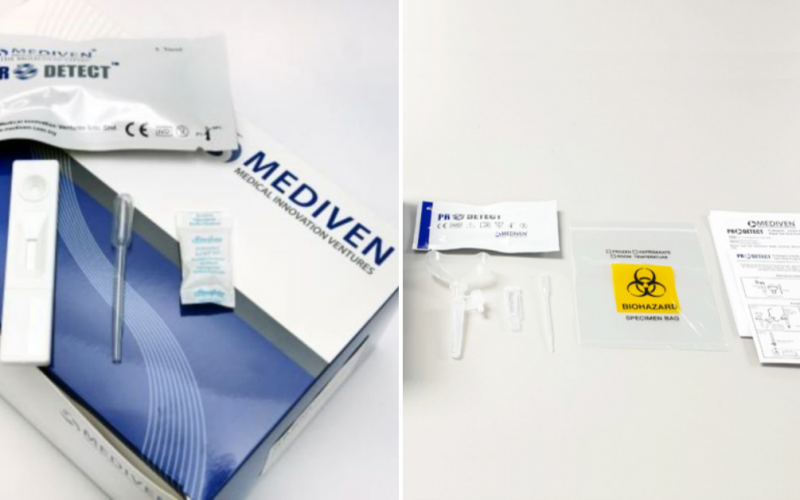
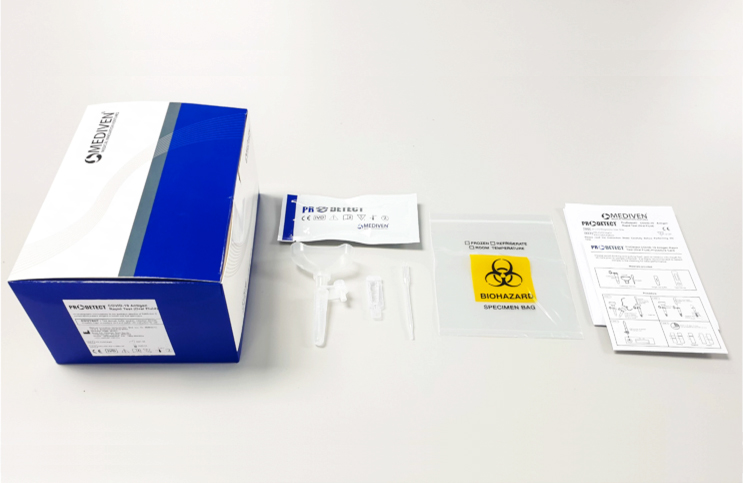
But with so many different brands now available for purchase through supermarkets, convenience stores and pharmacies, it is paramount to know which brands you can trust when it comes down to accuracy. And in the case of the ProDetect® COVID-19 Antigen Rapid Test manufactured by Malaysian company Medical Innovation Ventures Sdn Bhd (Mediven®), the European Society for external quality assessment (EsFEQA) has determined that it possesses a remarkable 100% accuracy rate from its External Quality Assessment Programme (EQAP).

As reported by BERNAMA, Mediven®’s ProDetect® COVID-19 Antigen Rapid Test is able to produce reliable COVID results in under 20 minutes and works on detecting all variants of concern (VOC) currently in transmission, including Delta and Omicron. And results obtained from the assessment using the kit have shown a high concordance (100 per cent) with Real-Time PCR results.
Recognition was well-received
In speaking with the Malaysian news agency, Mediven Operation’s Director Dr Lim Li Sze said that the company is proud of the accolade awarded to its product and reiterates their company’s commitment to quality.
“The COVID-19 rapid test serves as a valuable initial screening tool. As confirmed in the EQAP, our ProDetect® test kit retains its quality and consistency in providing a high level of accuracy.
As we see the cases rising by the day, it is crucial for early detection, isolation, and treatment of those infected and to prevent the further spread of COVID-19 infection.” he said.
The glowing commendation has also been warmly received by Mediven’s Executive Director, Ariff Ismail.
“Our achievement in the EQAP assessment serves as a testament, in recognition of the dedication and ingenuity of the team at Mediven®, as we strive to deliver cutting-edge diagnostics technology from bench side to bedside.” he said.
As many as 122 clinics and hospitals from 13 states and federal territories across West and East Malaysia that had been utilising the ProDetect® brand of test kits participated in the EQAP assessment, with participants all hailing from public hospitals and health clinics under each State’s Health Department.
Presently, Mediven produces two lines of ProDetect® test kits, one for use in a medical setting and another for use at home. The former uses either one of two types of samples, namely, oral fluid and nasopharyngeal swab. The latter uses either saliva or nasal samples.
For more news like this, follow us on Facebook by tapping here!